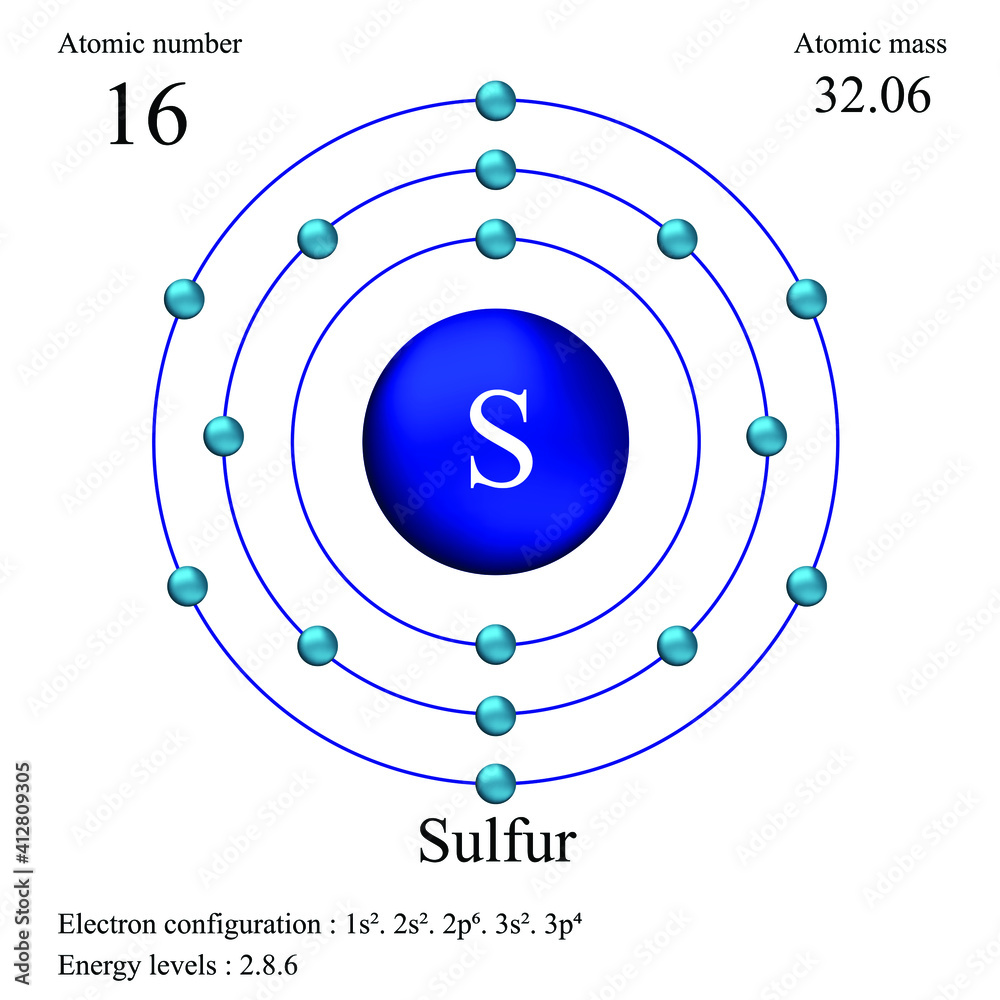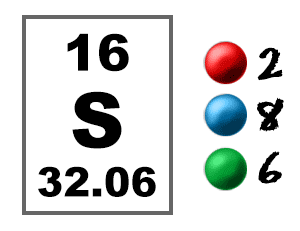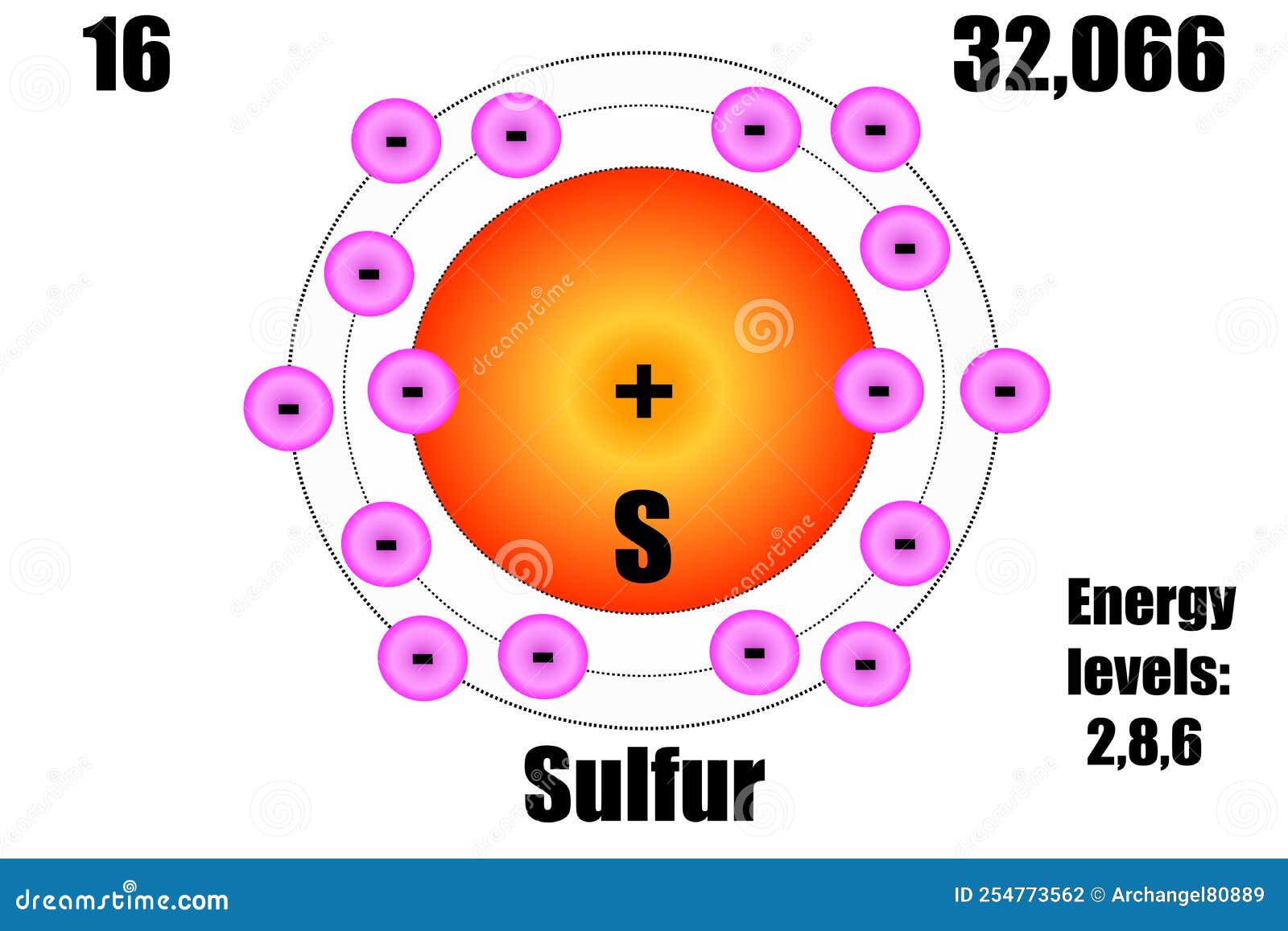
Sulfur Atom, with Mass and Energy Levels. Stock Vector - Illustration of formula, chemical: 254773562

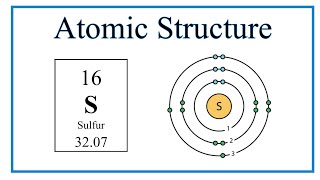
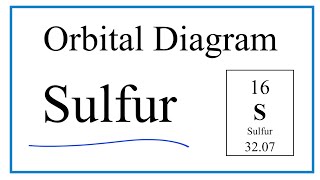
How to Write the Orbital Diagram for Sulfur (S)? | Electron configuration, Aufbau principle, Diagram
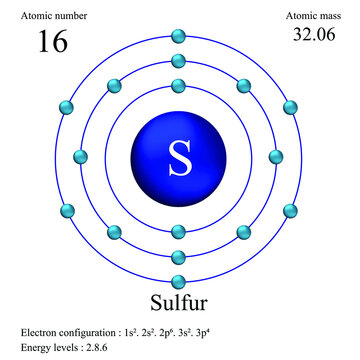
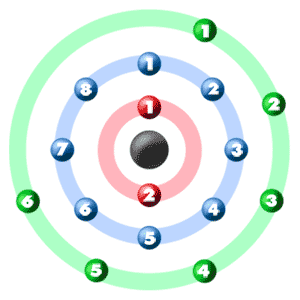
Sulfur atomic structure has atomic number, atomic mass, electron configuration and energy levels. Stock Vector | Adobe Stock

Sulphur has an Atomic number 16 and a Mass of 32. State the Number of Protons and Neutrons in the Nucleus of Sulphur. Give a simple Diagram to show the Arrangement of

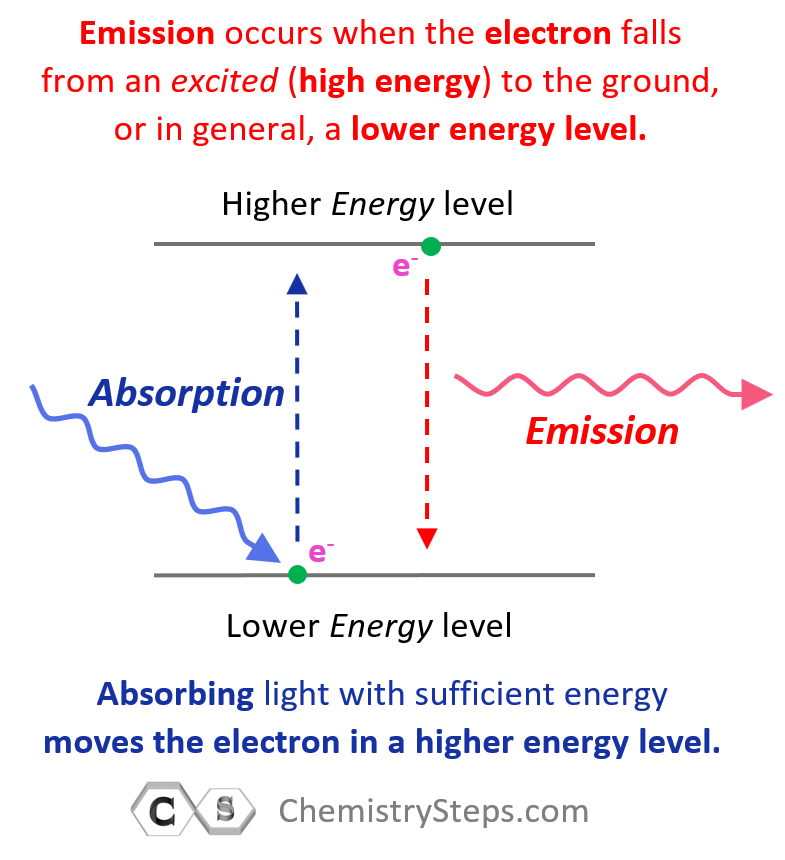
Question Video: Deducing the Energy Level Diagram Representing the Change from a Sulfur Atom to a Sulfur Ion | Nagwa

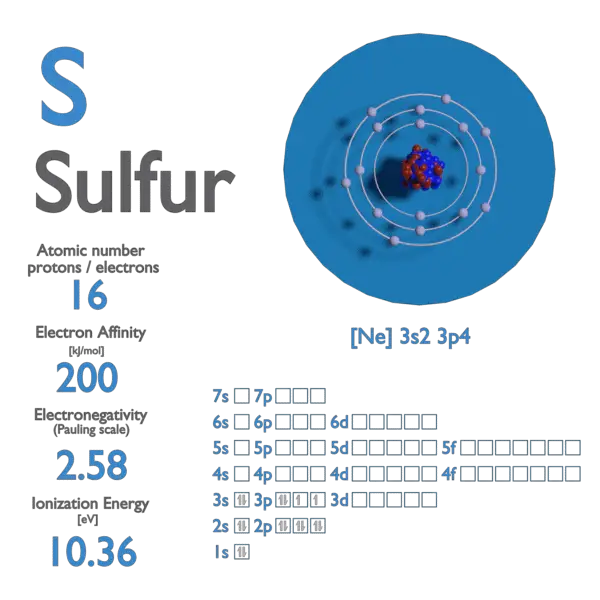
Draw the electron configuration of a ground state sulfur atom (Z = 16) showing the arrangement of electrons among the orbitals and the electron spins. What is the spin multiplicity of a