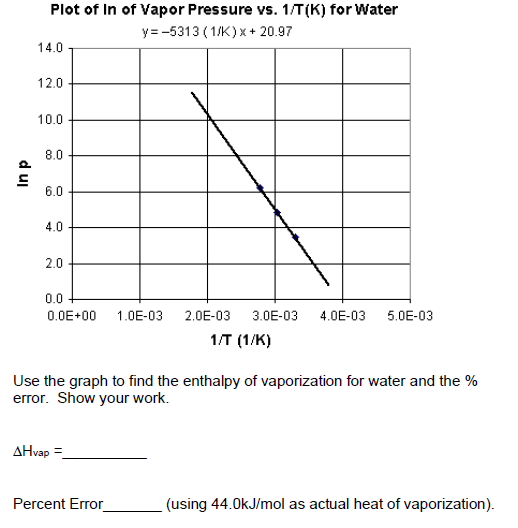
SOLVED: 7 The enthalpy of vaporization of water at 100.0-C is 40.68 kJ mol. What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 %C
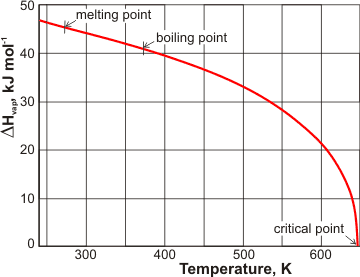
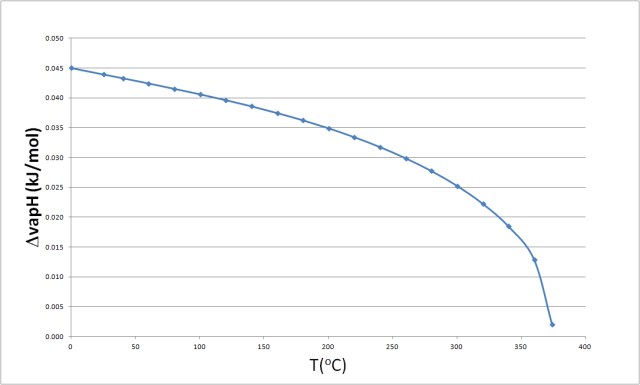
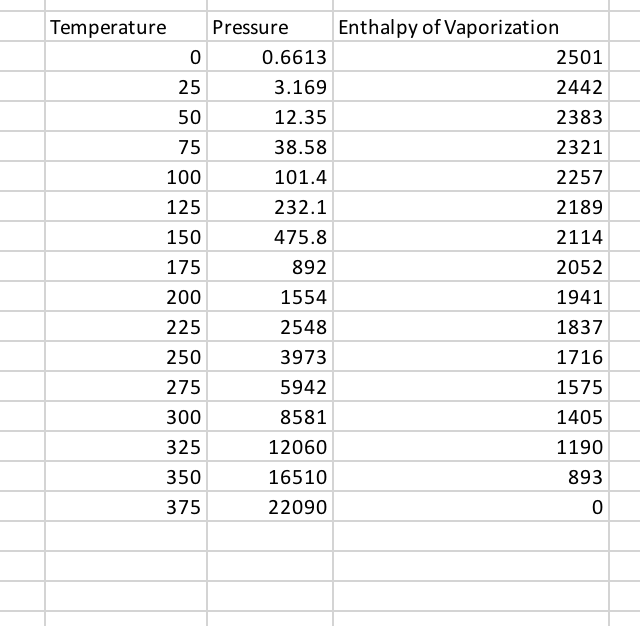
Molar enthalpy of vaporization of water from triple to critical points. | Download Scientific Diagram
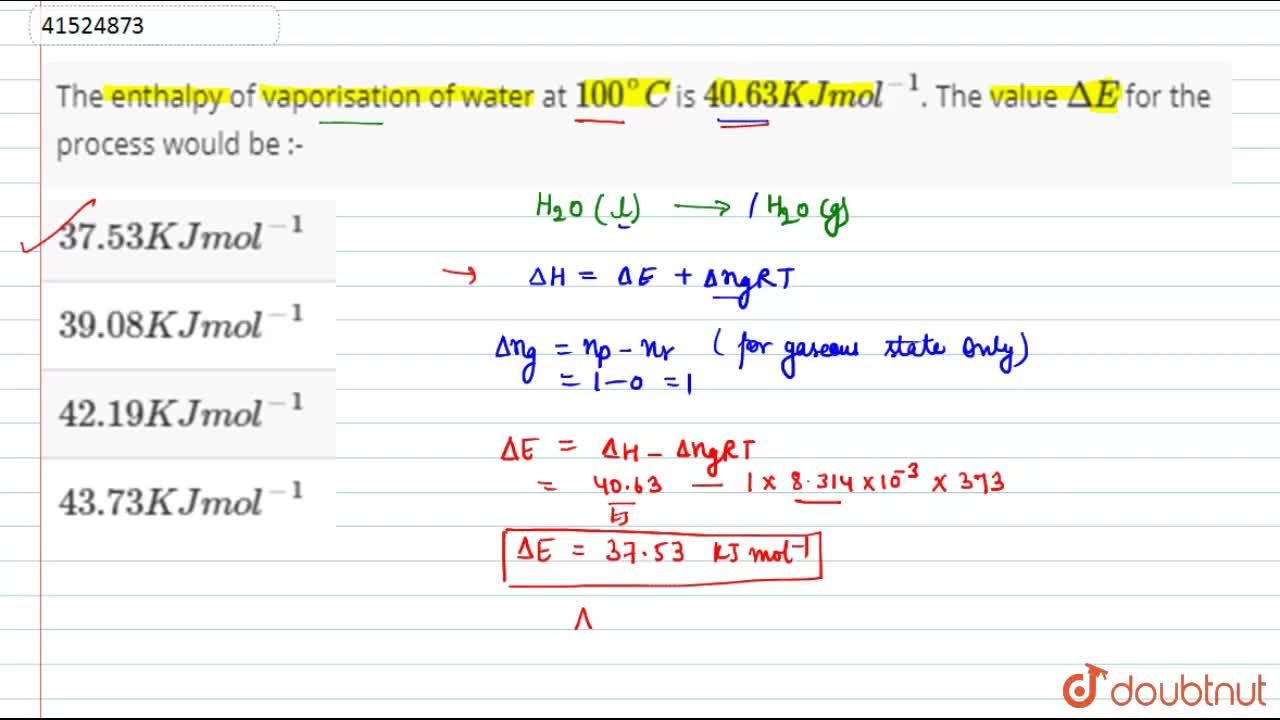
The enthalpy of vaporisation of water at 100^(@)C is 40.63 KJ mol^(-1). The value Delta E for the process would be :-
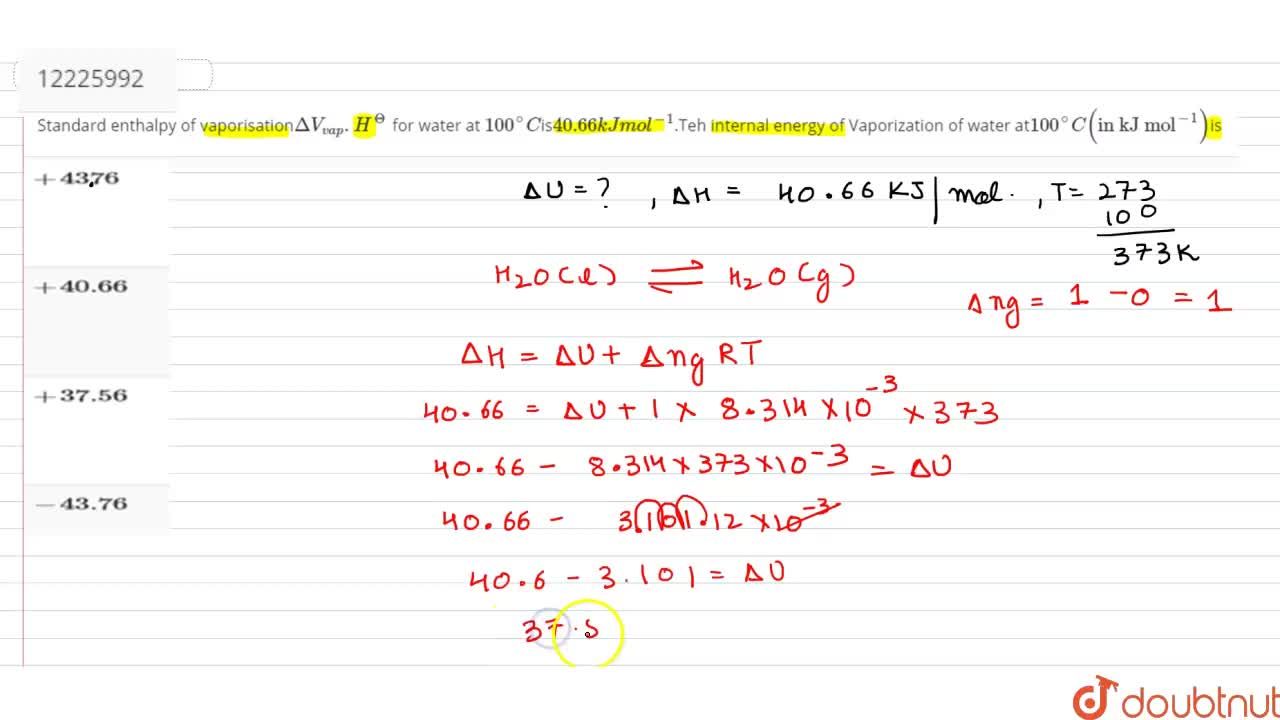
Standard enthalpy of vaporisationDeltaV(vap).H^(Theta) for water at 100^(@)Cis40.66kJmol^(-1).Teh internal energy of Vaporization of water at 100^(@)C("in kJ mol"^(-1))is

Standard enthalpy of vapourisation ΔvapH^ (0) for water at 100 is 40.66 kJmol^-1 . The internal energy of vapourisation of water at 100 (in kJmol^-1 ) is:

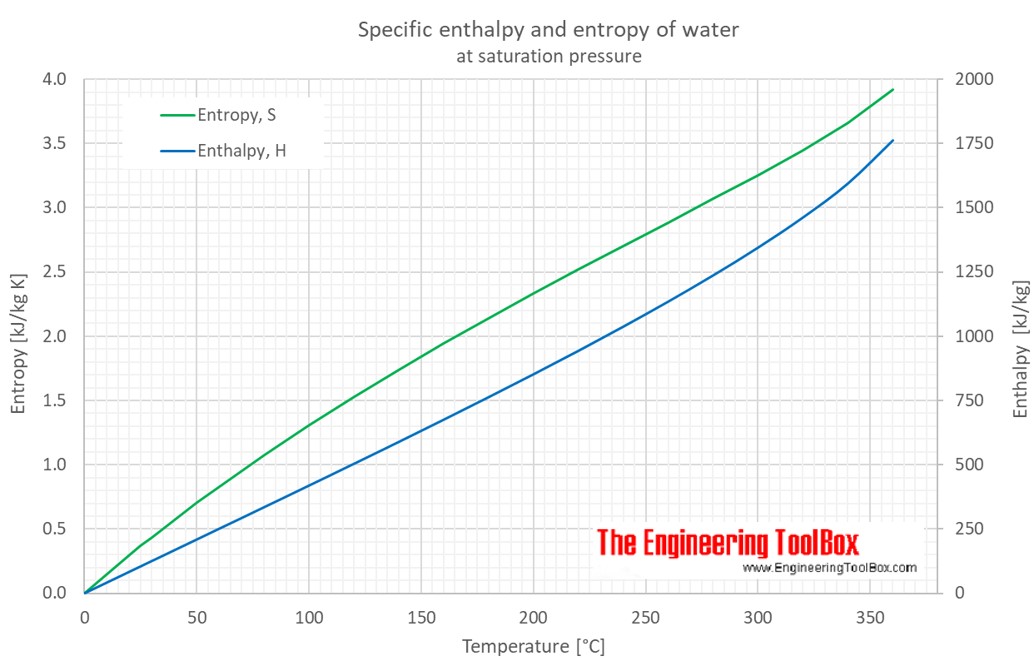
thermodynamics - Does adding salt to water decrease the latent heat of vaporization? - Physics Stack Exchange