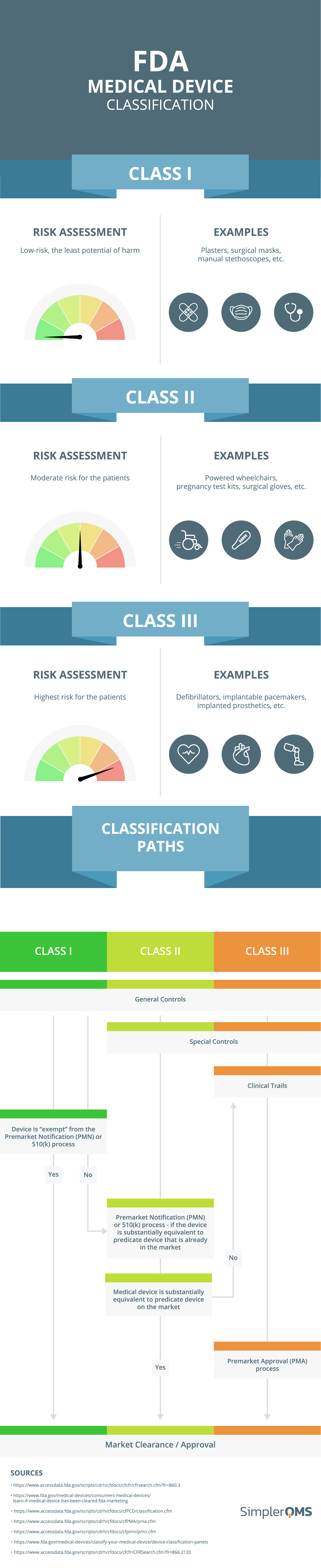
What are the Essential Requirements for Medical Device CE Marking? - Medical Device Academy Medical Device Academy

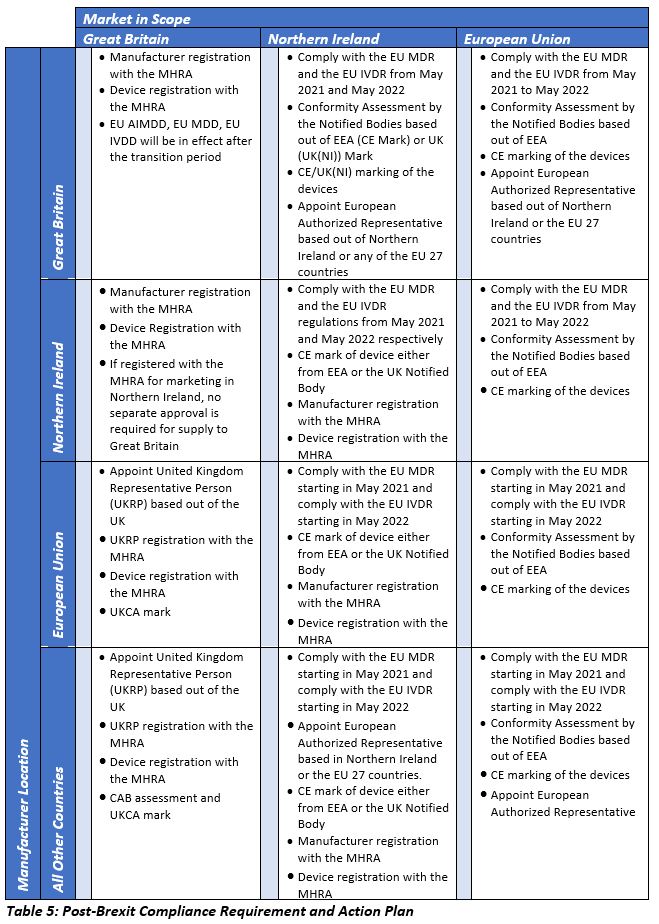
The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine

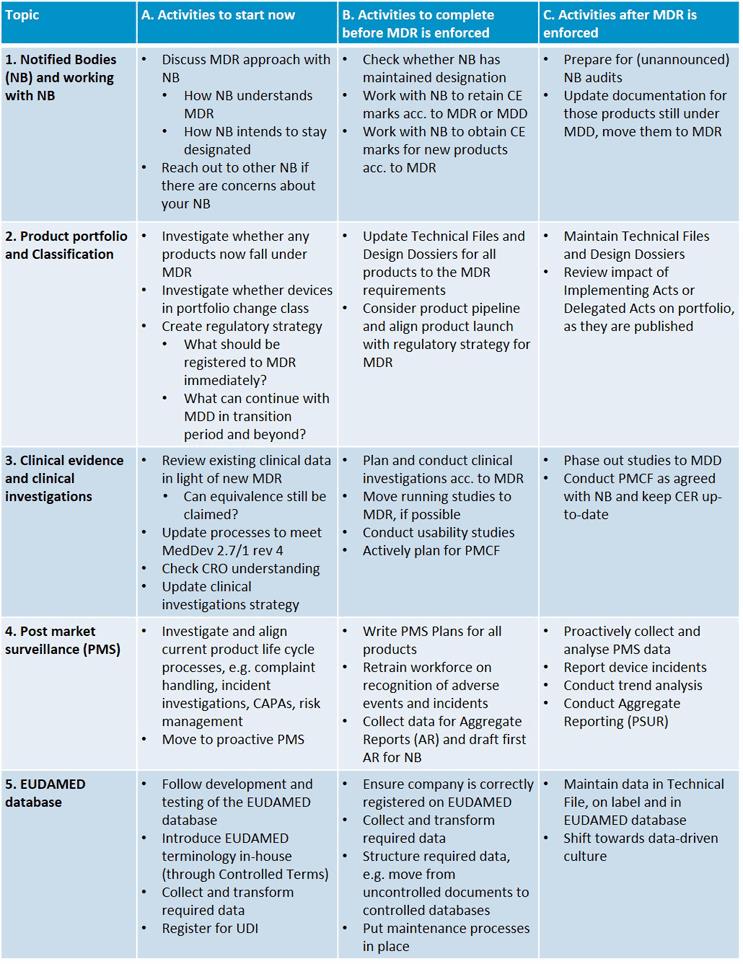
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products